On a quest to automate manual portions of the cell therapy production process, Multiply Labs is arming itself with two more pieces of industry-leading tech. The new kit comes on board a little less than a year after the robotics outfit unveiled a consortium aimed at building a robotic manufacturing system to make cell therapies on an industrial scale.
After recruiting an initial team that included Cytiva and the University of California, San Francisco (UCSF), Multiply Labs is adding major players in Thermo Fisher Scientific and Charles River Laboratories, the companies said Thursday.
With Multiply in charge of robotics and UCSF covering the cell manufacturing process itself, Cytiva, Thermo Fisher and Charles River will help pave the way for automation of bioreactors, incubators and quality control testing, respectively.
Multiply's goal is to ultimately automate the cell therapy production process end to end, Fred Parietti, co-founder and CEO, said in an interview. In the meantime, the consortium aims to demo the progress it's made so far at UCSF by the end of the year, he said.
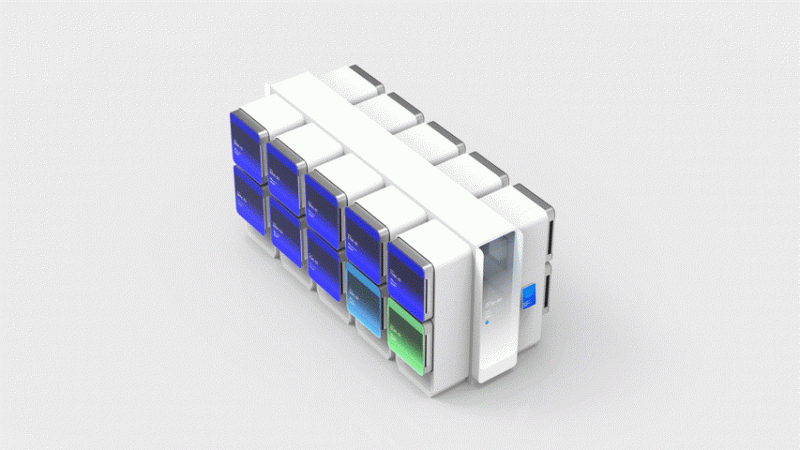
The consortium’s manufacturing system comprises a robotic cluster, where mechanical arms flanked by cube-shaped modules slide and pivot to move materials through production stages.
Controlled by cloud-based software, the cluster would also allow parts of the production process to run simultaneously as opposed to consecutively, which is more common today, Parietti told Fierce Pharma last year.
The cluster itself, with a configuration of 2x5 cube modules on each side, stands about nine feet tall and takes up just 150 square feet, Parietti said.
Because there are no humans inside the cluster—only a pair of robotic arms—the configuration functions as its own clean room, curbing potential contamination, he added.
The consortium is “based on the realization that we’re much better working together,” Parietti said, adding that if everybody “tried to reinvent the wheel … we’d still be here 10 years later.”
Cytiva is working with Multiply Labs to automate the operation of its Xuri bioreactors, with the goal to let robots handle the process and reduce physical touch points.
Thermo Fisher is providing its Heracell VIOS incubators, also with the goal to automate the equipment’s operation with Multiply Labs’ technical expertise.
Quality control, meanwhile, is where Charles River comes in. In addition to reducing room for human error, automation of Charles River's quality control testing platforms could significantly shorten the manufacturing process, the companies said.
As hundreds of developers propel their cell and gene prospects through the clinic, traditional manufacturing—which can take “hours of manual manipulation of cells and consumables”—simply isn’t cutting it, Multiply Labs noted in a release.
“There is a gigantic mismatch between demand and supply in cell therapy,” Parietti explained. Manufacturing processes for currently approved cell therapies like CAR-Ts already struggle to keep up, and, with hundreds more clinical prospects moving through trials, “it’s clear that you need a new technology,” Parietti said. “We just believe that robotics technology is both faster and more efficient.”